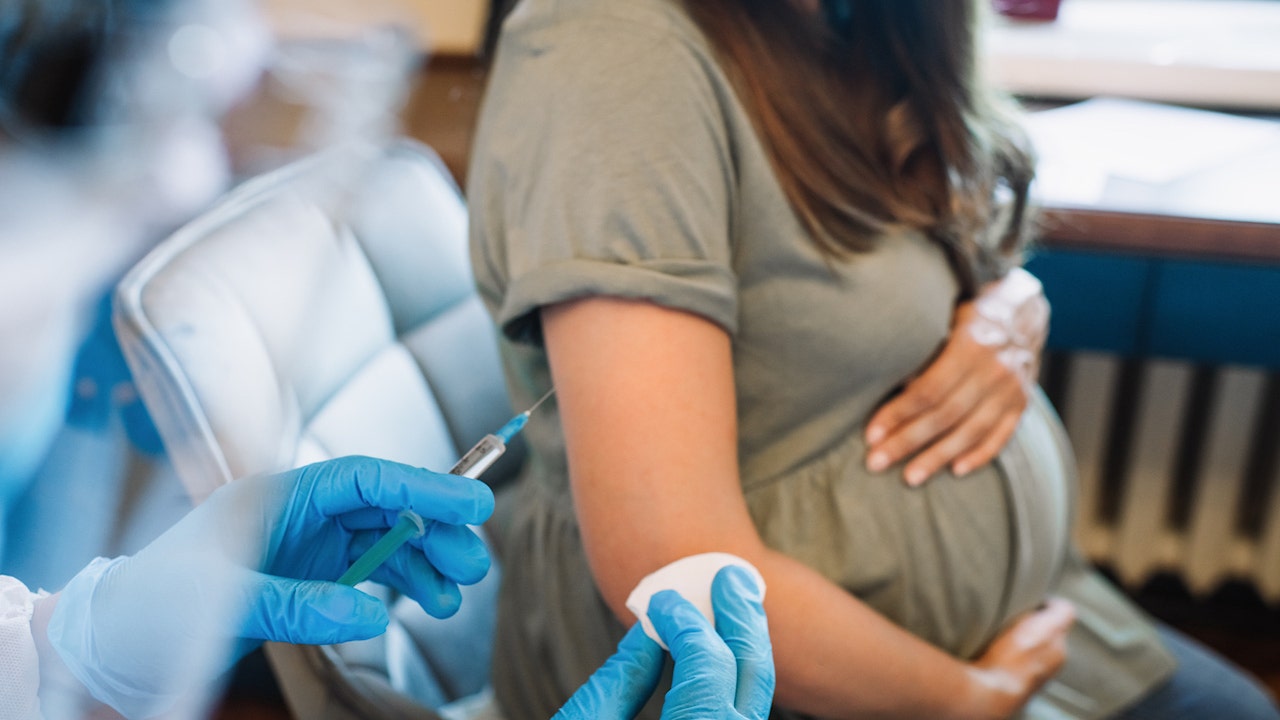
The Food and Drug Administration (FDA) has approved the first vaccine for pregnant women to prevent RSV in infants, the agency announced on Monday.
Abrysvo has been approved as a maternal vaccine to prevent lower respiratory tract disease (LRTD) in babies from birth through 6 months of age, according to the FDA’s press release.
The single-dose vaccine is administered via injection from 32 and 36 weeks of gestation.
FIRST-EVER RSV VACCINE APPROVED BY FDA FOR ADULTS 60 AND OVER
This news followed the FDA’s May announcement of the approval of Abrysvo to prevent LRTD in people 60 years of age and older.
RSV (respiratory syncytial virus) can cause an infection of the lungs and respiratory tract. It is very common among children — in fact, most are infected with the virus before 2 years of age.
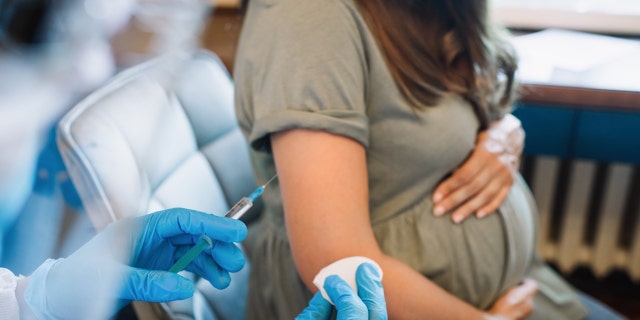
The Food and Drug Administration (FDA) has approved the first vaccine for pregnant women to prevent RSV in infants, the agency announced Monday. (iStock)
High-risk groups, including babies 12 months and younger, older adults, those who have heart and lung disease and immunocompromised people are more susceptible to serious illness, according to the Mayo Clinic’s website.
RSV is the world’s most frequent cause of lower respiratory tract illness, per the FDA.
It is the leading cause of infant hospitalization in the U.S.
“ABRYSVO’s approval as the first and only maternal immunization to help protect newborns immediately at birth through six months from RSV marks a significant milestone for the scientific community and for public health,” said Annaliesa Anderson, PhD, senior vice president and chief scientific officer at Pfizer, in a company press release.

“ABRYSVO’s approval as the first and only maternal immunization to help protect newborns immediately at birth through six months from RSV marks a significant milestone for the scientific community and for public health,” said Annaliesa Anderson, PhD, senior vice president and chief scientific officer at Pfizer. (REUTERS/Carlo Allegri/File Photo)
“Today, a long-sought-after goal to deliver a maternal vaccine that will help protect infants six months of age or younger — when they are at greatest risk of possible serious consequences from RSV — has been achieved.”
In most adults and older, healthy children, RSV only causes mild symptoms similar to the common cold, which typically don’t require medical attention.

“RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” said Peter Marks, M.D., PhD, director of the FDA’s Center for Biologics Evaluation and Research. (Getty Images)
“RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” said Peter Marks, MD, Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, in the FDA’s press release.
“This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease,” he added.
COVID VACCINES AND BOOSTERS SHOWN TO PROTECT PREGNANT WOMEN AND NEWBORNS: ‘TRANSFERRED PROTECTION’
The FDA’s approval comes after promising clinical studies that measured the “efficacy, safety and immunogenicity of the vaccine against LRTD and severe LRTD due to RSV in infants born to healthy individuals vaccinated during pregnancy,” Pfizer stated in its release.
The results of these studies were published in The New England Journal of Medicine in April 2023.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Of the 3,500 pregnant women who received the vaccine, there was more than an 81% reduced risk of their infants developing severe lower respiratory tract disease within the first three months of birth, compared to the women who received placebos.

RSV is the world’s most frequent cause of lower respiratory tract illness, per the FDA, and is the leading cause of infant hospitalization in the U.S. (iStock)
Among pregnant women who received the vaccine, the most common side effects were muscle pain, headache, nausea and pain at the injection site.
CLICK HERE TO GET THE FOX NEWS APP
There was a slightly higher occurrence of preeclampsia, low birth weight, preterm birth and jaundice in infants of mothers who received Abrysvo compared to the placebo group.